Laboratoire de Chimie Moléculaire et Thio-organique
Site en évolution : Le LCMT évolue et devient l'Institut CARMeN, suite à sa fusion avec le laboratoire COBRA.Recherche au LCMT
Les équipes de recherche au LCMT
Publications
Les publications du LCMT
Conférences
Les séminaires du LCMT
Groupe Thierry LEQUEUX
COORDINATEURPr Thierry Lequeux, directeur du LCMT
|
MEMBRES
|
AXES DE RECHERCHE
DOMAINES D’APPLICATIONS
|

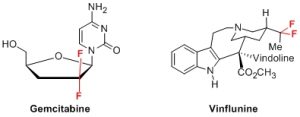
Les thématiques du groupe sont centrées sur la synthèse de petites molécules fluorées («briques moléculaires») pour l’élaboration de biomolécules plus sélectives. En effet, l’introduction d’atome(s) de fluor au sein de molécules hydrocarbonées modifie de façon considérable leurs propriétés physico-chimiques et leurs réactivités. La gemcitabine (Eli Lilly) et la vinflunine (Pierre Fabre), utilisés en chimiothérapie, illustrent l’apport du fluor dans le domaine pharmaceutique. Les trois principales thématiques du groupe concernent (1) la synthèse de nucléosides modifiés (fluorosulfures) (2) la préparation d’isostères de peptides (fluoroalcènes) (3) la préparation d’analogues de phosphates (difluorométhylphopshonates).
A – Synthèse de nucléosides fluorés et sulfurés
Mise au point de méthodes générales pour préparer des dithiocarbonates de fluoroalkyle. Application des méthodologies à la préparation de précurseurs de nucléosides modifiés (inhibiteurs d’enzymes).
- Synthesis of 2′,3′-dideoxy-2′-fluoro-4′-thionucleosides from a fluoroxanthate
- Synthesis and biological evaluation of fluorinated acyclothionucleosides
- Synthesis of 2,3-trans disubstituted tetrahydrofurans through sequential xanthate radical addition-substitution reaction.
B – Synthèse de fluoroalcènes comme isostères de peptides
Mise au point de méthodes de synthèse d’analogues de peptides à partir de sulfones fluorées. Préparation d’isostères de peptides renfermant un lien fluorovinylique. Application à la préparation d’insecticides, de dipeptides, d’hormones juvéniles d’insectes et d’inhibiteurs de mono-amine-oxydase. La stratégie employée est basée sur la réaction de Julia-Kocienski, réaction de fluoro-oléfination développée au laboratoire de Caen.
- Julia-Kocienski Fluoroolefination: A convenient one-step synthesis of fluoroethylidene derivatives
- Scope and limitations of the Julia-Kocienski reaction with fluorinated sulfonylesters
- Modified Julia fluoroolefination: selective preparation of fluoroalkenoates
C – Les Difluoromethylene phosphonates comme isostères de phosphates
Dans cette partie il a été mis au point la préparation d’un nouveau précurseur de radicaux libres et anion phosphonodifluorométhyle, sans utiliser les HCFC. Le développement de voies de synthèse de fluorométhylphosphonates par voies carbanionique et radicalaire est reporté. Application à la préparation d’un analogue d’état de transition des enzymes TP, en peu d’étapes à partir d’un phosphonosulfure.
- A difluorosulfide as a freon-free source of phosphonodifluoromethyl carbanion
- Syntheses of -Hydroxy-,-difluoromethylphosphonates by Oxacycle Ring-Opening Reactions
- Efficient synthesis of fluorophosphonylated alkyles by ring-opening reaction of cyclic sulfates
- Radical conjugated addition (RCA): addition of dialkyl phosphonodifluoromethyl radical onto unsaturated ketones
- Synthesis of fluorophosphonylated acyclic nucleotide analogues via copper (I)-catalyzed Huisgen 1,3-dipolar cycloaddition
The themes of the group are focused on the synthesis of fluorinated small molecules (« molecular bricks ») for the preparation of fluorinated biomolecules. Indeed, the introduction of fluorine atom(s) in hydrocarbon molecules significantly alters their physicochemical properties and reactivities. Gemcitabine (Eli Lilly) and Vinflunine (Pierre Fabre), used in chemotherapy, illustrate the contribution of fluorine in the pharmaceutical field.

A – Fluoroorgano compounds containing sulfur : synthesis
Development of general syntheses of fluoroalkyl dithiocarbonates. Applications to the preparation of modified nucleosides precursors (enzymes inhibitors)
- 1- Synthesis of 2′,3′-dideoxy-2′-fluoro-4′-thionucleosides from a fluoroxanthate
- 2- Synthesis and biological evaluation of fluorinated acyclothionucleosides
- 3- Synthesis of 2,3-trans disubstituted tetrahydrofurans through sequential xanthate radical addition-substitution reaction.
B – Synthesis of fluoroalkenes
Fluoroolefins are well known as precursors of biological active compounds and have been used in the fields of pheromones, herbicides and also as new medicines. Their syntheses based on Wittig or related reactions are limited to terminal fluoroalkenes, fluoro-unsaturated esters, or 1-fluoro-1-arylmethylidene derivatives. Some efforts have done during the last decade to prepare a variety of fluoroalkenes bearing alkyl or functionalized alkyl chain, to obtain enzyme inhibitors or peptide mimics. The most efficient strategies are the installation of the carbon-carbon double bond through a concerted elimination pathway from fluorosulfoxydes, fluorosilylacetates, fluorocarboxylates, or through a chemical modification of fluorovinylsulfones and fluoroalkenoates. To solve industrial problems (high cost or unavailability of reagents, tedious syntheses), we have developed different approaches to prepare fluoroolefins.
Development of methods for the synthesis of peptides analogues from fluorosulfones. Preparation of peptide isosteres. Application to the preparation of insect juvenile hormones and MonoAmineOxydase (MAO) inhibitors.
-
- 1- Julia-Kocienski Fluoroolefination: A convenient one-step synthesis of fluoroethylidene derivatives
- 2- Scope and limitations of the Julia-Kocienski reaction with fluorinated sulfonylesters
- 3- Modified Julia fluoroolefination: selective preparation of fluoroalkenoates
C – Difluoromethylene phosphonates: mimics of phosphates
Due to the limitation of methylenephosphonates as biological models of phosphates, difluoromethylenephosphonates emerge as stable isopolar phosphate mimics. Several methods for the syntheses of difluorophosphonates, analogues of important classes of phosphates, have been reported in the literature. For example, inhibitors of purine nucleoside phosphorylase were prepared via the direct alkylation of the diethoxyphosphosphono-difluoromethyllithium. These substrates were 18- to 96- fold more active than their corresponding methylenephosphonate.
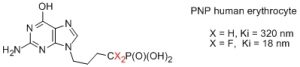
Inhibitor of purine nucleoside phosphorylase
Kondo’s and Burton’s groups have developed different methods for the direct introduction of the difluoromethylenephosphonate function, using the corresponding organometallic reagents, prepared from the dialkyl difluoro- and bromofluoro-methylphosphonates. These starting materials are readily obtained in good yields from chlorodifluoromethane or dibromodifluoromethane. However, due to environmental regulation, these methods are now limited and will be not available in a near future.
Development of new carbanionic routes to fluorométhylphosphonates, isosteric compounds of phosphates. Application to the preparation of an analogue of enzyme TP transition state.
-
-
- 1- A difluorosulfide as a freon-free source of phosphonodifluoromethyl carbanion
- 2- Syntheses of -Hydroxy-,-difluoromethylphosphonates by Oxacycle Ring-Opening Reactions
- 3- Efficient synthesis of fluorophosphonylated alkyles by ring-opening reaction of cyclic sulfates
- 4- Radical conjugated addition (RCA): addition of dialkyl phosphonodifluoromethyl radical onto unsaturated ketones
- 5-Synthesis of fluorophosphonylated acyclic nucleotide analogues via copper (I)-catalyzed Huisgen 1,3-dipolar cycloaddition
-
Brevets
Utilisation de dérivés de monomères issus de l’isosorbide comme substitut des dérivés du bis-phénol
Ho, A.-H.; Lequeux, T.; Pham, T. N.; Ely, G; Sotto, G.- G-Pharma/CNRS
Brevet Français 29/07/2016, FR3031976 A1
Fluorophosphonyl compounds, composition containing same and use thereof for restoring teeth
Lequeux, T.; Madec, P.-J.; Pham, T.-N.; Zulauf, A.
Brevet Monde, 2013 WO 2013/105049 A1
Preparation of monofluoroalkenes via a direct olefin formation from carbonyl compounds and metalated fluoro-heterocyclic surfones
S. Pazenok, J.-P. Demoute, S. Zard, T. Lequeux Brevet Europe, 2002, WO 0240459.
Publications sélectionnées
Selective preparation of tetrasubstituted fluoroalkenes by fluorine-directed oxetane ring-opening reactions
Clément Fontenelle, Thibault Thierry, Romain Laporte, Emmanuel Pfund, Thierry Lequeux
Beilstein J. Org. Chem, 2020, 16, pp.1936 – 1946.
doi: 10.3762/bjoc.16.160
hal-02922851
Difluorophosphonylated allylic ether moiety as a 2′-modification of RNA-type molecules: synthesis, thermal, and metabolic studies
Pfund, E.; Dupouy, C.; Rouanet,S.; Legay, R.; Lebargy, C.; Vasseur, J.-J.; Lequeux, T.
Org. Lett. 2019, 21, 4803−4807
DOI:10.1021/ acs.orglett.9b01689
hal-02168041v1
Photoinduced non–stabilized azomethine ylide formation for the preparation of fluorine containing pyrrolidines
Thierry, T.; Lebargy, C., Pfund, E.; Lequeux, T.
J. Org. Chem. 2019, 84 (9), 5877-5885.
DOI: 10.102/acs.joc.9b00244
Radical allylation: (E)-selective radical conjugate addition-elimination reaction from Morita-Baylis-Hillman adducts
Lebargy, C.; De Schutter,C.; Legay, R.; Pfund,E.; Lequeux,T.
Synlett 2018, 29(01), 46-50.
DOI: 10.1055/s-0036-1590922jo
Synthesis of fluoropyrrolidines and (fluoroalkyl)pyrrolidines
Pfund, E.; Lequeux, T.
Synthesis 2017, 49, 3848–3862.
DOI: 10.1055/s-0036-1589078
Synthesis of 5,5-difluoro-5-phosphono-pent-2-en-1-yl nucleosides as potential antiviral agents
Chevrier, F.; Chamas, Z.; Lequeux,T.; Pfund, E.; Andrei, G.; Snoeck, R.; Roy, V.; Agrofoglio, L. A.
RSC Adv. 2017, 7, 32282–32287.
DOI: 10.1039/c7ra05153k
Access to Fluoropyrrolidines by Intramolecular Aza-Michael Addition Reaction
Le Guen,, C.; Tran Do, M.-L.; Chardon, A.; Lebargy, C.; Lohier, J.-F.; Pfund, E.; Lequeux T.
J. Org. Chem. 2016, 81, 6714-6720.
DOI:10.1021/acs.joc.6b01363
Synthesis and characterization of innovative well- defined difluoro-phosphonylated-(co)polymers by RAFT polymerization
Ho, H.-T.; Coupris, J.; Pascual, S.; Fontaine, L; Lequeux, T.; Pham, T.-N.
Polym. Chem. 2015, 6, 4597-4604.
DOI: 10.1039/C5PY00690B
Synthesis of Fluorine Containing 3,3-Disubstituted Oxetanes and Alkylidene Oxetanes
Laporte, R.; Prunier, A.; Pfund, E.; Roy,R.; Agrofoglio, L. A.; Lequeux, T.
Eur. J. Org. Chem. 2015, 3121-3128.
DOI: 10.1002/ejoc.201500172
Synthesis of fluorinated and CF3 substituted alkenes through the modified Julia olefination: an update
Pfund, E.; Lequeux, T.; Gueyrard, D.
Synthesis 2015, 1534-1546.
DOI: 10.1055/s-0034-1380548
Fluorinated hydroxypiperidines as selective -glucosidase inhibitors
Leguen, C.; Mena, T.; Ortiz Mellet, C.; Gueyrard, D.; Pfund, E.; Lequeux, T.
Org. Biomol. Chem., 2015, 13, 5983-5996.
DOI: 10.1039/C5OB00721F
Stereoselective formation of (Z)-2-fluoroalkenoates via Julia Kocienski reaction of aldehydes with pyrimidinyl-fluorosulfones
Larnaud, F.; Pfund, E.; Linclau, B.; Lequeux, T.
Tetrahedron, 2014, 70, 5632-5639.
DOI: 10.1016/j.tet.2014.06.081
Synthesis of fluorinated agonist of sphingosine-1-phosphate receptor 1
Aliouane, L. ;Chao, S.; Brizuela, L.; Pfund, E.; Cuvillier, O.; Jean, L.; Renard, P.-Y.; Lequeux. T.
Bioorganic & Medicinal Chemistry 2014, 22, 4955-4960.
DOI: org/10.1016/j.bmc.2014.06.038
Fluorophosphonylated monomers for dental applications
Derbanne, M.; Zulauf, A.; LeGoff, S.; Pfund, E.; Sadoun, M.; Pham, T.-N.; Lequeux, T.
Org. Process. Res. Dev. 2014, 18, 1010-1019.
DOI: 10.1021/op500108m
Synthesis of substituted exo-glucals via a modified Julia olefination and identification as selective -glucosidase inhibitors
Habib S.; Larnaud F.; Pfund E.; Mena Barragán T.; Lequeux T.; Ortiz Mellet C.; Goekjian P. G.; Gueyrard D.
Org. Biomol. Chem. 2014, 690-699. (IF = 3.56)
DOI: 10.1039/c3ob41926f
Aza-Michael access to fluoroalkylidene analogues of biomolecules
Prunier, A.; Calata, C.; Legros, J.; Maddaluno, J.; Pfund, E.; Lequeux, T.
J. Org. Chem. 2013, 78, 8083-8097. (IF = 4.56)
DOI: org/10.1021/jo401356j
Radical Conjugate Addition of Ambiphilic Fluorinated Free Radicals
C. De Schutter, E. Pfund, T. Lequeux
Tetrahedron, 2013, 69, 5920-5926.
DOI: 10.1016/j.tet.2013.05.006. (IF = 3.02)
Synthesis of fluorinated exo-Glycals through modified Julia olefination
S. Habib, F. Larnaud, E. Pfund, T. Lequeux, B. Fenet, P. Goekjean, D. Gueyrard
Eur. J. Org. Chem., 2013, 1872-1875. (IF = 3.32)
DOI: 10.1002/ejoc.201201719
Ready Synthetic Access to Enantiopure Allylic (F)-Branched Fluoroalkenes
F. Larnaud, J. Malassis, E. Pfund, B. Linclau, and T. Lequeux
Organic Lett., 2013, 15, 2450-2453. (IF = 5.86)
Fluorophosphonylated Nucleoside Derivatives as New Series of Thymidine Phosphorylase Multisubstrate Inhibitors
S. Diab, C. De Schutter, M. Muzard, R. Plantier-Royon, E. Pfund, T. Lequeux
J. Med. Chem., 2012, 55, 2758-2768. (IF = 5.207)
Convergent synthesis of functionalized uoroallylamines by the Julia Kocienski reaction
C. Calata, E. Pfund, T. Lequeux
Tetrahedron, 2011, 67, 1398-1405. (IF = 2.89)
Synthesis of fluorophosphonylated acyclic nucleotide analogues via copper(I)-catalyzed Huisgen 1-3 dipolar cycloaddition
S. Diab, A. Hienzch, C. Lebargy, S. Guillarme, E. Pfund, T. Lequeux
Org. Biomol. Chem., 2009, 4481-4490. (IF = 3.55)
Toward the Synthesis of Benzothiazolyl Fluoroaminosulfones
C. Calata, E. Pfund, T. Lequeux
J. Org. Chem., 2009, 74, 9399-9405. (IF = 3.95)
Efficient Synthesis of Fluorophosphonylated Alkyles by Ring-Opening Reaction of Cyclic Sulfates
S. Diab, A. Sene, E. Pfund, T. Lequeux
Organic Letters, 2008, 10, 3895-3898.
Synthesis and biological evaluation of Þuorinated acylthionucleosides
S. Gouault-Bironneau, A. Sene, J.-M. Catel, T. Lequeux
Journal of Fluorine Chemistry, 2008, 129, 848-851.
Modified Julia fluoroolefination: selective preparation of fluoroalkenoates
E. Pfund, C. Lebargy, J. Rouden, T. Lequeux
Journal of Organic Chemistry, 2007, 72, 7871-7877.
Synthesis of 2,3-trans Di-substituted Tetrahydrofurans through Sequential Xanthate Radical Addition-Substitution Reactions
L. Jean-Baptiste, S. Yemets, R. Legay, T. Lequeux
Journal of Organic Chemistry, 2006, 71, 2352-2359.
INTERNATIONNALES
Bruno Linclau (Université de Southampton)
M. Carmen Ortiz Mellet (Université de Séville)
NATIONALES
David Gueyrard / Peter Goekjean (Université de Lyon UCBL)
Jacques Maddaluno (Université de rouen – COBRA)
Pierre-Yves Renard (Université de Rouen – COBRA)
LOCALES
Nhan Pham (LCMT)
Jacques Rouden (LCMT)