Laboratoire de Chimie Moléculaire et Thio-organique
Site en évolution : Le LCMT évolue et devient l'Institut CARMeN, suite à sa fusion avec le laboratoire COBRA.Recherche au LCMT
Les équipes de recherche au LCMT
Publications
Les publications du LCMT
Conférences
Les séminaires du LCMT
Groupe Annie-Claude Gaumont
|
|
|
MEMBRES
|
AXES DE RECHERCHE
DOMAINES D’APPLICATIONS
|
L’objectif de ce groupe de recherche est de créer de nouvelles molécules organo-phosphorées et sulfurées d’une part, en vue d’étudier leurs applications comme intermédiaires de synthèse sélectifs notamment en synthèse asymétrique et organométallique et, d’autre part, pour leurs activités potentielles dans le domaine du médicament, de l’agrochimie ou des matériaux.
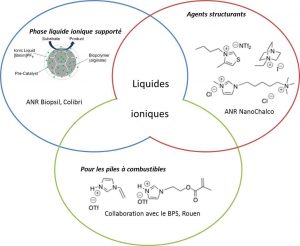
Un autre aspect de ce groupe est la synthèse à façon de liquides ioniques pour des applications variées.
Chimie du Phosphore
- Synthèse de phosphure borane
J. Am. Chem. Soc. 2011, 133, (16), 6472-‐6480
- Etude de mécanismes réactionnels
L’objectif de cette thématique de recherche est de tirer profit des outils de la chimie physique organique pour étudier les mécanismes réactionnels de procédés précédemment développés dans notre laboratoire, en particulier :
– la chimie des phosphido boranes
– l’hydrophosphination énantiosélective
– la catalyse de phosphines et l’aminocatalyse
- Couplage C-P catalysé au cuivre
Chem. Commun. 2012, 4088
Chimie du soufre
- Ions sulfénates précurseurs de sulfoxydes
Org. Lett., 2011,3170
- Synthèses multicomposants : N,S hétérocycles
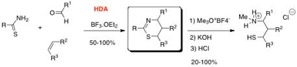
Tetrahedron, 2012, 68, 9016
Nouveaux milieux de types liquides ioniques
Phosphorus Chemistry
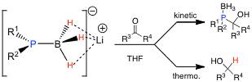
A multinuclear NMR study shows that the deprotonation of diphenylphosphine-borane by n-BuLi in THF leads to a disolvated lithium phosphido-borane Ph2P(BH3)Li of which Li+ is connected to the hydrides on the boron and two THF molecules rather than to the phosphorus. This entity behaves as both a phosphination and a reducing agent, depending on the kinetic or thermodynamic control imposed to the reaction medium. Density functional theory computations show that H2P(BH3)Li exhibits a ditopic character (the lithium cation can be in the vicinity of the hydride or of the phosphorus). It explains its dual reactivity (H- or P-addition), both routes going through somewhat similar six-membered transition states with low activation barriers.

Our research aims to take advantage of physical organic chemistry tools to investigate the reactions mechanisms of processes previously developed in our laboratory, in particular:
– Phosphido boranes chemistry;
– Enantioselective hydrophosphination;
– Phosphine catalysis and aminocatalysis.
Sulfur Chemistry
Ionic liquids
Publications 2022
Recovery of functionalized rubber from waste tires by radical devulcanization
Noël J.N., Gaumont A.C., Pilard J.F., Dez I.; ACS Sustainable Chem. Eng. 2022, 10, 1, 159–165
DOI: 10.1021/acssuschemeng.1c05228
Publications 2021
Novel ionic conducting composite membrane based on polymerizable ionic liquids
Yaroslav L. Kobzar, Ghania Azzouz, Hashim Albadri, Jocelyne Levillain, Isabelle Dez,
Annie-Claude Gaumont, Laurence Lecamp, Corinne Chappey, Stéphane Marais, Kateryna Fatyeyeva
Polymers 2021, 13, 3704.
DOI: 10.3390/polym13213704
Method for producing an aromatic diether and corresponding methods for producing polyaryletherketones
Bussi, Philippe; Le Guillaume; Gaumont Annie-Claude; Al Badri Hashim
Brevet Français (2019) FR1915347
Brevet Monde PCT (2021), WO 2021/123688 A1
hal-03366275
Functionalization of [2.2]paracyclophanes via a reductive sulfanylation reaction
Damien Deschamps, Jean-François Lohier, Annie-Claude Gaumont, Christopher J. Richards, Stéphane Perrio
J. Org. Chem. 2021, 86, 507–514.
DOI: 10.1021/acs.joc.0c02235
Publications 2020
Synthesis of functionalized polymers through devulcanization from waste containing elastomers
Dez, Isabelle; Gaumont, Annie-Claude; Noël, Jean-Nicolas
PCT Int. Appl. (2020), WO 2020169589 A1 2020082
Visible-light-mediated C–H alkylation of pyridine derivatives
Fatima Rammal, Di Gao, Sondes Boujnah, Annie-Claude Gaumont, Aqeel Hussein, Sami Lakhdar
Organic Letters, American Chemical Society, 2020, 22 (19), pp.7671-7675.
doi: 10.1021/acs.orglett.0c02863
hal-03011210
Photochemical C–H Silylation and Hydroxymethylation of Pyridines and Related Structures: Synthetic Scope and Mechanisms
Fatima Rammal, Di Gao, Sondes Boujnah, Aqeel Hussein, Jacques Lalevée, Sami Lakdhar
ACS Catalysis, American Chemical Society, 2020 10 (22), pp.13710-13717.
doi:10.1021/acscatal.0c03726
hal-03011244
Visible-light-mediated access to phosphate esters
A. Inial, F. Morlet-Savary, J. Lalevée, A−C Gaumont, S. Lakhdar
Org. Lett. 2020, 22, 4404–4407.
doi:10.1021/acs.orglett.0c01409
hal-03011254
Taming the reactivity of phosphiranium salts: site‐selective C‐centered ring opening for direct synthesis of phosphinoethylamines
Julien Gasnot, Clément Botella, Sébastien Comesse, Sami Lakhdar, Carole Alayrac, Annie‐claude Gaumont, Vincent Dalla, Catherine Taillier
Angewandte Chemie International Edition, 2020, 59 (29), pp.11769-11773.
doi: 10.1002/anie.201916449
hal-02899688
Access to stable quaternary phosphiranium salts by P-alkylation and P-arylation of phosphiranes
Julien Gasnot, Clément Botella, Sébastien Comesse, Sami Lakhdar, Carole Alayrac, Annie-Claude Gaumont, Vincent Dalla, Catherine Taillier
Synlett 2020, 31 (09), pp.883-888.
doi : 10.1055/s-0040-1708000
hal-02899695
A straightforward synthesis of N-substituted ureas from primary amides
Nathalie Saraiva Rosa, Thomas Glachet, Quentin Ibert, Jean-François Lohier, Xavier Franck, Vincent Reboul
SYNTHESIS, Georg Thieme Verlag, 2020, 52(14): 2099-2105.
doi :10.1055/s-0040-1707103
hal-02569464
Design of iodinated radioligands for SPECT imaging of central human 5-HT4R using a ligand lipophilicity efficiency approach
Victor Babin, Benjamin Tournier, Audrey Davis, Emmanuelle Dubost, Gilbert Pigrée, Jean-François Lohier, Vincent Reboul, Thomas Cailly, J.-P. Bouillon, Philippe Millet, Frédéric Fabis
Bioorganic Chemistry, Elsevier, 2020, 96, pp.103582.
doi.org/10.1016/j.bioorg.2020.103582
hal-02466870
Metal-Free Visible-Light-Mediated Aromatization of 1,2-Dihydronaphthalenes
Fatima Rammal, Annie-Claude Gaumont, Sami Lakhdar
European Journal of Organic Chemistry, Wiley-VCH Verlag, 2020, 10, 1482-1485.
doi: 10.1002/ejoc.201901410⟩
hal-02479941
Publications 2019
Iodonitrene in action: direct transformation of amino acids into terminal diazirines and 15 N 2-diazirines and their application as hyperpolarized markers
T. Glachet, H. Marzag, N. Rosa, J. Collel, G. Zhang, W. Warren, X. Franck, T. Theis, V. Reboul
J. Am. Chem. Soc. 2019, 141(34), 13689-13696.
DOI: 10.1021/jacs.9b07035
hal-02276187
Terminal diazirines enable reverse polarization transfer from 15 N 2 singlets
G. Zhang, J. Colell, T. Glachet, J. Lindale, V. Reboul, T. Theis, W. Warren
Angew. Chem. Int. Ed., 2019, 58 (32), 11118-11124.
DOI: 10.1002/anie.201904026
hal-02173605
Visible–light–mediated alpha–phosphorylation of N–aryl tertiary amines through the formation of electron–donor–acceptor Complexes: synthetic and mechanistic studies
V. Quint, N. Chouchène, M. Askri, J. Lalevée, A.-C. Gaumont, S. Lakhdar
Org. Chem. Front. 2019, 6, 41–44.
DOI: 10.1039/C8QO00985F
hal-02172993
Diphenyliodonium Ion/Et3N Promoted C(sp2)–H Radical Phosphorylation of Enamides
S. Pal, A-C. Gaumont, S. Lakhdar, I. Gillaizeau
Org. Lett. 2019, 21 (14), 5621-5625.
DOI : 10.1021/acs.orglett.9b01963
hal-02172975
Iodinated Polystyrene for Polymeric Charge Transfer Complexes: Toward High-Performance Near-UV and Visible Light Macrophotoinitiators
A.Baralle, P. Garra, B. Graff, F. Morlet-Savary, C. Dietlin, J–P. Fouassier, S. Lakhdar, J. Lalevée
Macromolecules 2019, 52, 3448–3453.
DOI:10.1021/acs.macromol.9b00252
hal-02172933
The facile dearomatization of nitroaromatic compounds using lithium enolates of unsaturated ketones in conjugate additions and (4+2) formal cycloadditions
K. Pasturaud, B. Rkein, M. Sanselme, M. Sebban, S. Lakhdar, M. Durandetti, J. Legros, I. Chataigner
Chem. Commun., 2019, 55, 7494–7497.
DOI: 10.1039/C9CC02924A
hal-02156509v1
Charge transfer complexes as dual thermal and photochemical polymerization initiators for 3D printing and composites synthesis
D. Wang, P. Garra, S. Lakhdar, B. Graff, J-P. Fouassier, H. Mokbel, M. Abdallah, J. Lalevée,
ACS Appl. Polym. Mater. 2019, 1, 561-570.
DOI: 10.1021/acsapm.8b00244
hal-02172933
Evidence of phosphonium-carbenium dications formation in superacid: key precursors of fluorinated phosphine oxides
Castelli, U.; Lohier, J.-F.; Drukenmüller, I.; Mingot, A.; Bachman, C.; Alayrac, C.; Marrot, J.; Stierstorfer, K.; Kornath, A.;Gaumont, A.-C.; Thibaudeau, S.
Angewandte Chemie, Inter. Ed, 2019, 1369-1374.
DOI: 10.1002/ange.201811032
hal-02159927
Hydrophobization of chitosane films by surface grafting with fluorinated polymer brushes
Lepoittevin, B.; Elzein, T.; Dragoe, D.; Bejjani, A.; Lemée, F.; Levillain, J.; Bazin, P.; Roger, P.; Dez, I.
Carbohydrate Polymers, 2019, 205, 437-446.
DOI : 10.106/j.carbpol.2018.10.044
hal-01917571
Late-stage sulfoximination: improved synthesis of the anticancer drug candidate Atuveciclib
Glachet, T.; Franck, X.; Reboul, V.
Synthesis, 2019, 51(04): 971-975.
DOI: 10.1055/s-0037-1610316
hal-01926262
Publications 2018
One-pot synthesis of aryl- and alkyl S-perfluoralkylated NH-sulfoximides from sulfides
Chaabouni, S.; Lohier, J.-F.; Barthelemey, A.-L.; Glachet, T.; Anselmi, E.; Dagousset, G.; Diter, P.; Pégot, B.; Magnier, E.; Reboul, V.
Synthetic Methods, 2018, 24 (64), 17006-17010.
DOI: 10.1002/chem.201805055
Sulfinate-organocatalyzed (3+2) annulation of allenyl sulfones with 1,1-dicyano olefins in the presence of a quaternary ammonium phase transfer agent.
Martzel, T.; Lohier, J.-F.; Gaumont, A.-C.; Brière, J.-F.; Perrio, S.
Adv. Synth. Catal., 2018, 360, 2696–2706.
DOI: 10.1002/adsc.201800466
Synthesis and identification of aryl and alkyl gem-dilithium phosphide-boranes: a boost to the chemistry of phosphandiides
Harrison-Marchand, A.; Guang, J.; Duwald, R.; Maddaluno, J.; Oulyadi, H.; Lakhdar, S.; Gaumont, A.-C.
Chem. Eur. J. 2018, 24(26), 6717-6721.
DOI: 10.1002/chem.201800742
Construction of isoxazolidin-5-ones with a tetrasubstituted carbon center: enantioselective conjugate addition mediated by phase-transfer catalysis
Cadart, T.; Levacher, V.; Perrio, S.; Brière,J.-F.
Adv. Synth. Catal., 2018, 360, 1499–1509.
DOI: 10.1002/adsc.201800009
Insight in the alginate Pd-ionigel- Application to the Tsuji-Trost reaction
Vittoz, P. F.; El Siblani, H.; Bruma, A.; Rigaud, B.; Sauvage, X.; Fernandez, C.; Vicente, A.; Barrier, N.; Malo, S.; Levillain, J.; Gaumont, A.-C.; Dez, I.
ACS, Sustainable Chemistry & Engineering 2018, 6, 5192-5197.
DOI:10.1021/acssuschemeng.7b04832
Publications 2017
Direct syn addition of two silicon atoms to a C≡C triple bond by Si-Si activation: access to reactive disilytated olefins
Ahmad, M.; Gaumont, A.-C.; Durandetti, M.; Maddaluno, J.
Angew. Chem. Inter. Ed. 2017, 56 (9), 2464-2468.
DOI:10.1002/anie.201611719
Mechanistic investigations of reactions of the frustrated Lewis pairs (triarylphosphines/B(C6F5)3) with Michael acceptors
Dupré, J.; Gaumont, A.-C.; Lakhdar, S.
Org. Lett. 2017, 19(3), 694-697.
DOI:10.1021/acs.orglett.6b03868
Transition metal-free stereospecific access to (E)-(1-fluoro-2-arylvinyl)phosphine borane complexes
Rousée, K.; Pannecoucke,X.; Gaumont,A.-C.; Lohier,J.-F.; Morlet-Savary,F.; Lalevée, J.; Bouillon,J.-P.; Couve-Bonnaire, S.; Lakhdar, S.
Chem. Commun., 2017,53, 2048-2051
DOI: 10.1039/C6CC09673E
Synthesis of methionine-derived endocyclic sulfilimines and sulfoximines
Marzag, H.; Schuler, M.; Tatibouët, A.; Reboul, V.
Eur. J. Org. Chem. 2017, 896-900.
DOI : 10.1002/ejoc.201601515
Mechanistic investigation of the NH-sulfoximination of sulfide. Evidence for λ6-sulfanenitrile intermediates
Lohier, J.-F.; Glachet, T.; Marzag, H.; Gaumont, A.-C.; Reboul, V.
Chem. Commun. 2017, 53, 2064-7.
DOI: 10.1039/c6cc09940h
Unique Reactivity of -Substituted Electron-Deficient Allenes using Sulfinate Salts as Lewis Base Organocatalysts
Martzel, T.; Lohier, J.-F.; Gaumont, A.-C.; Brière, J.-F.; Perrio, S.
Adv. Synth. Catal. 2017, 356(1), 96-106.
DOI: 10.1002/adsc.201600929
How do phosphinates react with unactivated alkenes under organic photocatalyzed conditions? Substrate scope and mechanistic insights
Fausti, G.; Morlet-Savary, F.; Lalevée, J.; Gaumont, A.-C.; Lakhdar, S.
Chem. Eur. J. 2017, 23, 1-6.
DOI: 10.1002/chem.201604683
Selected as a Hot Paper by the Editor of Chemistry a European Journal
Publications 2016
Enantioselective Phase-Transfer Catalyzed -Sulfanylation of Isoxazolidin-5-ones: An Entry to 2,2-Amino Acid Derivatives
Cadart, T.; Berthonneau, C.; Levacher, V.; Perrio, S.; Brière, J.-F.
Chem. Eur. J. 2016, 22, 15261-15264.
DOI: 10.1002/chem.201603910
Metal-free synthesis of 6-phosphorylated phenanthridines: synthetic and mechanistic insights
Noël-Duchesneau, L.; Lagadic, E.; Morlet-Savary, F.; Lohier, J.-F.; Chataigner, I.; Breugst, M.; Lalevée, J.; Gaumont, A.-C.; Lakhdar, S.
Org. Lett. 2016, 18, 5900-5903.
DOI: 10.1021/acs.orglett.6b02983
Highlighted in Synfacts : Synthesis of 6-Phosphorylated Phenanthridines, 2017, 13, 140.
Metal-free, visible light-photocatalyzed synthesis of benzo[b]phosphole oxides: synthetic and mechanistic investigation
Quint, V.; Morlet-Savary, F.; Lohier, J.-F.; Lalevée, J.; Gaumont, A.-C.; Lakhdar, S.
J. Am. Chem. Soc. 2016, 138, 7436-7441.
DOI: 10.1021/jacs.6b04069
Highlighted in Synfacts, 2016, 12, 862.
Access to constrained fluoropseudopeptides via ring-closing metathesis of fluoroalkenes
Guerin, D.; Dez, I.; Gaumont, A. C.; Pannecoucke, X.; Couve-Bonnaire, S.
Org. Lett., 2016, 18 (15), 3606-3609.
DOI: 10.1021/acs.orglett.6b01631
Convenient access to meso benzylic bisalkynes
Bulman, P. C.; Richard, S. G.; Harvey, J.; Gaumont, A.-C.; Alayrac, C.; Slawin, A. M. Z.
Synlett, 2016, 27 (6), 961-964.
DOI : 10.1055/s-0035-1561318
Enantiomerically Pure [2.2]Paracyclophane-4-thiol: A Planar Chiral Sulfur-based Building Block Readily Available by Resolution with an Amino Acid Chiral Auxiliary
Vincent, A.; Deschamps, D.; Martzel, T.; Lohier, J.-F.; Richards, C. J.; Gaumont, A.-C.; Perrio, S.
J. Org. Chem., 2016, 81(9), 3961-3966.
DOI : 10.1021/acs.joc.6b00560
Room-temperature alkynylation of phosphine oxides with copper acetylides: practical synthesis of alkynylphosphine oxides
Gerard, P.; Veillard, R.; Alayrac, C.; Gaumont A.-C.; Evano, G.
Eur. J. Org. Chem. 2016, 633-638.
DOI: 10.1002/ejoc.201501440
Publications 2015
tert-Butyl Sulfoxides: Key Precursors for Palladium-Catalyzed Arylation of Sulfenate Salts
Gelat, F.; Lohier, J.-F.; Gaumont A.-C.; Perrio, S.
Adv. Synth. Catal., 2015, 357, 2011-2016.
DOI: 10.1002/adsc.201500368
Synthesis of 1,3 thiazines by a three-component reaction and their transformations into beta-lactam-condensed 1,3-thiazine and 1,4 thiazine derivatives
Peudru, F.; Lohier, J.-F.; Gulea, M.; Reboul, V.
Phosphorus, Sulfur, and Silicon and the Related Elements, 2015, 191, 220-229.
DOI: 10.1080/10426507.2015.1072191
Publications 2014
Biopolymer Supported Ionic Liquid Phase (bio-SILP) Ruthenium Catalyst for Olefin Metathesis
Clousier, N.; Filippi, A.; Borré, E.; Guibal, E.; Crévisy, C.; Caijo, F.; Mauduit, M.; Dez, I.; Gaumont, A.-C.
Chem. Sus. Chem. 2014, 7(4), 1040-1045.
DOI: 10.1002/cssc.201300804
Valorisation des déchets élastomères : du déchet pneumatique au polymère fonctionnel
Mouawia, A.; Nourry, A.; Gaumont, A.-C.; Pilard J.-F.; Isabelle Dez, I.
L’actualité chimique 2014 – n° 390- p.88-89.
Tuning morphology of GeS2 hybrid materials using Ionic Liquids as structuring agent
Mathiaud, R.; Courtheoux, L.; Silly, G.; Albadri, H.; Levillain, J.; Gaumont A.-C.; Ribes, M.; Pradel, A.
European Journal of Inorganic Chemistry 2014, 6232-6238.
DOI:10.1002/ejic.201402762
Silyl alkynylphosphine-boranes: key precursors of triazolylphosphines via tandem desilylation-Click chemistry
Veillard, R.; Bernoud, E.; Abdellah, I.; Lohier, J.-F.; Alayrac, C.; Gaumont, A.-C.
Org. Biomol. Chem. 2014, 12, 3635-3640.
DOI: 10.1039/C4OB00397G
Turning unreactive copper acetylides into remarkably powerful and mild alkyne transfer reagents by oxidative umpolung
Evano, G.; Jouvin, K.; Theunissen, C.; Guissart, C.; Laouiti, A.; Tresse, C.; Heimburger, J.; Bouhoute, Y.; Veillard, R.; Lecomte, M.; Nitelet, A.; Schweiser, S.; Blanchard, N.; Alayrac, C.; Gaumont, A.-C.
Chem. Commun. 2014, 50, (70), 10008-10018.
DOI: 10.1039/C4CC03198A
Methyllithiodithioacetate
Perrio, S.
In e-eros Electronic Encyclopedia of Reagents for Organic Synthesis, B. A. Charrette Ed. ;Wiley: 2014; pp 1-12.
DOI: 10.1002/047084289X.rm204.pub2
A copper-catalyzed variant of the Michaelis-Arbuzov reaction
Ballester, J.; Gatignol, J.; Schmidt, G.; Alayrac, C.; Gaumont, A.-C.; Taillefer, M.
ChemCatChem, 2014, 6, 1549-1552.
DOI: 10.1002/cctc.201301029
HAL: hal-01015701
Access to fluorinated lactams through ring-closing metathesis of reluctant fluoroalkenes promoted by appropriate substitution of a double bond
Guerin, D.; Gaumont, A.-C.; Dez, I.; Mauduit, M.; Couve-Bonnaire, S.; Pannecoucke, X.
ACS Catal., 2014, 4, 2374-2378.
DOI: 10.1021/cs500559p
HAL: hal-01015764
Reduction of S=O and SO2 to S, S-X to S-H, and P=O to P
Gaumont, A.-C.; Gulea, M.; Perrio, S.; Reboul, V.
In Gary A. Molander and Paul Knochel (Eds), Comprehensive Organic Synthesis, 2nd Edition, Volume 8, Oxford: Elsevier; 2014, pp. 535-563.
Metal-catalyzed synthesis of hetero-substituted alkenes and alkynes
Evano, G.; Gaumont, A.-C.; Alayrac, C.; Wrona, E. I.; Giguere, J. R.; Delacroix, O.; Bayle, A.; Jouvin, K.; Gatignol, J.; Silvanus, A. J.
Tetrahedron 2014, 70, 1529-1616.
DOI: 10.1016/j.tet.2013.11.073
HAL: hal-00993029
Publications 2013 et antérieures
Asymmetric three-component domino reaction: an original access to chiral nonracemic 1,3-thiazin-2-ones
Peudru, F.; Le Cavelier, F.; Lohier, J.-F.; Gulea, M.; Reboul, V.
Org. Lett. 2013, 15, 5710-5713.
DOI: 10.1021/ol4027446
An investigation of the asymmetric Huisgen « Click » reaction
Buttress, J. P.; Deschamps, D.; Lancelot, M.; Martin, J.-P.; Sheldon, A. I. G.; Alayrac, C.; Gaumont, A.-C.; Page, P. C. B.; Stephenson, G. R.
Synlett 2013,24, 2723-2729.
DOI: 10.1055/s00331340152
HAL: hal-00915673
Chiral non racemic sulfoxides by asymmetric alkylation of alkanesulfenates in the presence of a chiral ammonium phase transfer catalyst derived from Cinchona alkaloid
Gelat, F.; Gaumont, A.-C.; Perrio, S.
J. Sulfur Chem. 2013, 34, 596-605.
DOI: 10.1021/jp408455x
HAL: hal-00915656
Iron-salt-promoted highly regioselective alpha and beta hydrophosphination of alkenyl arenes
Routaboul, L.; Toulgoat, F.; Gatignol, J.; Lohier, J.-F.; Norah, B.; Delacroix, O.; Alayrac, C.; Taillefer, M.; Gaumont A.-C.
Chem. Eur. J., 2013, 19, 8760-8764.
DOI: 10.1002/chem.201301417
HAL: hal-00832386
Unprecedented synthesis of alkynyl phosphine-boranes through room-temperature oxidative alkynylation
Jouvin, K.; Veillard, R.; Theunissen, C.; Alayrac, C.; Gaumont, A.-C.; Evano, G.
Org. Lett. 2013, 15 (17), 4592-4595.
DOI: 10.1021/ol402197d
HAL: hal-00877877
Ligand exchange reaction on Au38(SR)24, separation of Au38(SR)23(SR’)1 regioisomers and migration of thiolates
Beqa, L.; Deschamps, D.; Perrio, S.; Gaumont, A.-C.; Knoppe, S. ; Bürgi, T.
J. Phys. Chem. C, 2013, 117, 21619-21625.
DOI: 10.1021/jp408455x »10.1021/jp408455x
HAL: hal-00903467
Copper-catalyzed synthesis of 1,3-butadienylphosphine oxides and chemoselective phosphoryl reduction to stereodefined 1,3-butadienylphosphines
Gatignol, J.; Alayrac, C.; Lohier, J.-F.; Ballester, J.; Taillefer, M.; Gaumont, A.-C.
Adv. Synth. Catal. 2013, 355, 2822-2826.
DOI: 10.1002/adsc.201300618/abstract »10.1002/adsc.201300618
HAL: hal-00924102
Hybrid macroporous Pd catalytic discs for 4- nitroaniline hudrogenation: contribution of the alginate-tetraalkylphosphonium ionic liquid support
Vincent, T.; Krys, P.; Jouannin, C.; Gaumont, A.-C.; Dez, I.; Guibal, E.
J. Organomet. Chem. 2013, 723, 90-97.
DOI: org/10.1016/j.jorganchem.2012.10.008
HAL: hal-00903438
Highly porous catalytic materials with Pd and ionic liquid supported on chitosan
C. Jouannin, C. Vincent, I. Dez, A.-C. Gaumont, T.Vincent, E. Guibal
J. Appl. Pol. Sci. 2013, 128 (5), 3122-3130.
DOI: 10.1002/app.38501
HAL: hal-00868539
Facile access to gamma-aminothiols from 1,3-thiazines via a microwave-assisted three component reaction
F. Peudru, R. Legay, J.-F. Loyer, V. Reboul, M.Gulea
Tetrahedron 2012, 68, 9016-9022.
DOI: org/10.1016/j.tet.2012.08.072
Stoichiometric and Catalytic Synthesis of Alkynylphosphines
E. Bernoud, R.Veillard, C. Alayrac, A.-C. Gaumont
Molecules 2012, 17, 14573-14587.
Influence of the structure of the onium iodide salts on the properties of modified montmorillonite
S. Livi, C. Dufour, A.-C. Gaumont, J. Levillain, T.-N. Pham
J. of Appl. Pol. Sci. 2012, 127 ( 5), 4015-4026.
DOI: 10.1002/app.3793710
Nitrophenol hydrogenation using Pd immobilized on ionic liquid-alginate spherical resins.
J. Czulak, C.Jouannin, T. Vincent, I. Dez, A.-C.Gaumont, E. Guibal
Separation Science and Technology 2012, 47, (14-15), 2166-2176.
DOI: 10.1080/01496395.2012.697521
Study of alginate-supported ionic liquid and Pd catalysts.
C. Jouannin, C. Vincent, I. Dez, A.-C.Gaumont, T. Vincent, E. Guibal, E.
Nanomaterials 2012, 2, (1), 31-53.
DOI: 10.3390/nano2010031
Synthetic methodologies for the preparation of beta-aminothiols.
G. Mercey, V. Reboul, M. Gulea, J. Levillain, A.-C. Gaumont
Eur. J. Org. Chem. 2012, 5423-5434.
DOI : 10.1002/ejoc.201200347
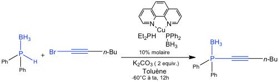
Neutral copper-phosphido-borane complexes: synthesis, characterization, and use as precatalysts in Csp-P bond formation
I. Abdellah, E. Bernoud, J.-F. Lohier, C. Alayrac, L. Toupet, C. Lepetit, A.-C. Gaumont
Chem. Commun. 2012, 48, (34), 4088-4090.
DOI: 10.1039/C2CC30723E
Asymmetric diastereoselective thia-hetero-Diels-Alder reactions of dithioesters
H. Dentel, I. Chataigner, J.-F. Lohier, M. Gulea
Tetrahedron 2012, 68, 2326-2335.
DOI: 10.1016/j.tet.2012.01.039
Synthesis of a [2.2] paracyclophane based planar chiral palladacycle by a highly selective kinetic resolution/C-H activation reaction.
N. Dendele, F. Bisaro, A.-C. Gaumont, S. Perrio, C. J. Richards
Chem. Commun. 2012, 48, 1991-1993.
DOI: 10.1039/C2CC16864B
Cyclic sulfenamide: versatile template for the synthesis of 1,4-benzothiazepines
C. Spitz, V. Reboul, P. Metzner
Tetrahedron Lett. 2011, 52, 6321-6324.
DOI: 10.1016/j.tetlet.2011.07.148
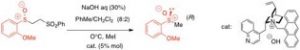
Organocatalytic asymmetric synthesis of sulfoxides from sulfenic acid anions mediated by a cinchona-derived phase transfer reagent
F. Gelat, J. Jayashankaran, J.-F. Lohier, A.-C. Gaumont, S. Perrio
Org. Lett. 2011, 13, 3170-3173.
DOI: 10.1021/ol2010962
Ph2P(BH3)Li: from ditopicity to dual reactivity
G. B. Consiglio, P. Queval, A. Harrison-Marchand, A. Mordini, J.-F. Lohier, O. Delacroix, A.-C. Gaumont, H. Gerard, J. Maddaluno, H. Oulyadi
J. Am. Chem. Soc. 2011, 133, (16), 6472-6480.
DOI: 10.1021/ja201760c
Synthesis and evaluation of a broad range of new chiral phosphine-carbene ligands for asymmetric hydrogenation
J. Passays, T. Ayad, V. Ratovelomanana-Vidal, A.-C. Gaumont, P. Jubault, E. Leclerc
Tetrahedron Asymmetry 2011, 22, (5), 562-574.
DOI: 10.1016/j.tetasy.2011.02.017
Copper-catalyzed synthesis of alkynylphosphine derivatives: unprecedented use of nucleophilic phosphorus compounds
Bernoud, E.; Alayrac, C.; Delacroix, O.; Gaumont A.-C.
Chem. Commun. 2011, 47, 3239-3241.
DOI: 10.1039/C0CC04645K
Palladium supported on alginate/ionic liquid highly porous monoliths: application to 4-nitroaniline hydrogenation
C. Jouannin, I. Dez, A.-C. Gaumont, J.-M. Taulemesse, T. Vincent, E. Guibal
Appl. Cat. B: Environemental 2011, 103, (3-4), 444-452.
DOI : 10.1016/j.apcatb.2011.02.008
Mechanistic insights into the Palladium-catalysed asymmetric phosphination of cyclo-hexenyltriflate
D. Julienne, O. Delacroix, J.-F. Lohier, J. S. de Oliveira-Santos, A.-C. Gaumont, A.-C.
Eur. J. Inorg. Chem. 2011, 2011, (16), 2489-2498.
DOI : 10.1002/ejic.201000987
Hetero-Diels-Alder reactions of new sulfonylsulfines generated from -substituted methylsulfones
H. Dentel, J.-F. Lohier, M. Gulea
ARKIVOC 2011, vi, 62-73.
Catalytic materials based on catalysts containing ionic liquid phase supported on chitosan or alginate: importance of the support
N. Clousier, R. Moucel, P. Naik, J.-P. Madec, A.-C. Gaumont, I. Dez, I.
C. R. Chimie 2011, 14, (7-8), 680-684.
DOI : 10.1016/j.crci.2010.08.004
First catalytic enantioselective version of a thia hetero-DielsAlder reaction with dithioesters
H. Dentel, I. Chataignier, F. Le Cavelier, M. Gulea
Tetrahedron Lett. 2010, 51, 6014-6017.
DOI: 10.1016/j.tetlet.2010.09.055
Mercaptophosphonate compounds as broad-spectrum inhibitors of the metallo–lactamases
P. Lassaux, M. Hamel, M. Gulea, H. Delbrück, P. S. Mercuri, L. Horsfall, D. Dehareng, M. Kupper, J.-M. Frère, K.Hoffmann, M. Galleni, C. Bebrone
J. Med. Chem. 2010, 53, 4862-4876.
DOI: 10.1021/jm100213c
Importance of the conditioning of the chitosan support in SILP catalysts : the palladium catalysed allylation reaction case
R. Moucel, K. Perrigaud, J.-M. Goupil, P.-J. Madec, S. Marinel, E. Guibal, A.-C. Gaumont, I. Dez
Adv. Synth. Cat., 2010, 352, 433-439.
DOI: 10.1002/adsc.200900515
An overview of the synthesis of alkenylphosphines
D. Julienne, O. Delacroix, A.C. Gaumont
Current Org. Chem., 2010, 14, 457-482.
DOI: 10.2174/138527210790601152
Synthesis of chiral thiazoline ligands tethered to a sulfur function and first immobilization of a thiazoline ligand
A. Betz, A.C. Gaumont, I. Dez, M. Gulea
Heteroatom Chem., 2010, 21, 242-249.
Alkenylphosphines: applications in synthesis, catalysis and coordination chemistry
D. Julienne, F. Toulgoat, O. Delacroix, A.C. Gaumont
Current Org. Chem., 2010, 14, 1195-1222.
Synthesis of 3-amino-thiochromanes from 4-benzyl 2-thiazolines, via an unprecedented intramolecular electrophilic aromatic substitution
G. Mercey, R. Legay, J.F. lohier, J. Sopkova-de Oliveira Santos, J. Levillain, A.C. Gaumont, M. Gulea
Org. Biomol. Chem., 2010, 8, 2520-2521.
DOI: 10.1039/c003050c
First study on the enantioselective palladium-catalyzed C-P cross-coupling reaction between an alkenyltriflate and a phosphine-borane
D. Julienne, O. Delacroix, A.C. Gaumont
CR Chim., 2010, 13 (8-9), 1099-1103.
DOI: org/10.1016/j.crci.2010.06.003
Thermoplastic starch plasticized by an ionic liquid
A. Sankri, A. Arhaliass, I. Dez, A.-C. Gaumont, Y. Grohens, D. Lourdin, I. Pillin, A. Rolland-Sabaté, Eric Leroy
Carbohydrate Polymers, 2010, 82, 256-263.
DOI: 10.1016/j.carbpol.2010.04.032<
Vers une chimie moléculaire verte
J.M. Campagne, F. Agbossou, T. a Ayad, O. Baudoin, G. Buono, I. Chataigner, C. Crévisy, I. Dez, M. Donnard, F. Fache, C. Feasson, A.C. Gaumont, L. Giordano, P. Hesemann, F. Marsais, M. Mauduit, V. Michelet, A. Mortreux, P. Phansavath, O. Piva, J.P. Roblin, J. Rouden, M. Taillefer, P. Y. Toullec, Tschamber T., V. Vidal
L’actualité chimique, 2010, 338-339, 15-27.
First C-P cross-coupling reaction of vinyltosylates with diphenylphosphine-borane : new access to Vinylphopshine-borane derivatives.
D. Julienne, O. Delacroix, A.C. Gaumont,
Phoshorus, Sulfur, Silicon & related Elements, 2009, 184, 846-856- Sur invitation N° spécial dédié au Pr Mikolajczyk
DOI : 10.1080/10426500802715551
Michael addition to a chiral non-racemic 2-phosphono-2,3-didehydrothiolane S-oxide
M. Gulea, M. Kwiatkowska, P. Lyzwa, R. Legay, A.C. Gaumont, P. Kielbasinski,
Tetrahedron : Asymmetry, 2009, 20, 293-297.
Versatile synthesis of secondary 2-amino thiols and/or their disulfides via thiazolinium salts
G. Mercey, J.F. Lohier, A.C. Gaumont, J. Levillain, M. Gulea
Eur. J. Org. Chem., 2009, 4357-4364
DOI:10.1002/ejoc.200900514
Thiazolinium ionic liquid: an efficient catalyst for the Mukaiyama Reaction
G. Mercey, D. Brégeon, C. Baudequin, F. Guillen, J. Levillain, M. Gulea, J-C. Plaquevent, A.-C. Gaumont
Tetrahedron Lett., 2009, 50, 7239-7241.
Mild and efficient access to lithium alkanesulfinates based on oxaziridine-promoted oxidation of thiolates
C. Caupène, C. Martin, M. Lemarié, S. Perrio et P. Metzner,
J. Sulfur Chem., 2009, 30, 338-345.
DOI : 10.1080/17415990902903009
Fluoride ion and phosphines as nucleophilic catalysts: synthesis of 1,4 benzothiazepines from cyclic sulfenamides
C. Spitz, J.-F. Lohier, J. Sopkova-de Oliveira Santos, V. Reboul and P. Metzner
J. Org. Chem. 2009, 74, 3936-3939.
Catalytic generation of cesium acetylide by CsF: synthesis of 1,3-benzothiazines from cyclic sulfenamides
C. Spitz, J.-F. Lohier, V. Reboul and P. Metzner
Org. Lett. 2009, 11, 2776-2779.
An Overview of the Chemistry of 2-Thiazolines
A.-C. Gaumont, M. Gulea, J. Levillain
Chem. Rev., 2009, 109 (3), pp 1371-1401.
DOI: 0.1021/cr800189z
Ionic liquids : new targets and media for amino acid and Peptide chemistry
J.-C. Plaquevent, J. Levillain, F Guillen, C. Malhiac, A.-C. Gaumont
Chemical Reviews, 2008, 108 (12), 5035-5060.
Efficient synthesis of primary 2-aminothiols from 2-aminoalcohols and
methyldithioacetate
G. Mercey, D Brégeon, A.-C. Gaumont, J. Levillain, M. Gulea
Tetrahedron Letters, 2008, 49, 6553-6555.
Convenient mild and selective hydrophosphination of functionalised alkenes: access to P,O
and P,S derivatives
B. Join, J.-F. Lohier, O. Delacroix, A.-C. Gaumont
Synthesis, 2008, 19, 3121-3125.
Pummerer type reactions in the (2-methylsulfanyl-2-phosphonyl) thiopyran 1-oxide
series
M. Denance, R. Legay, A.-C. Gaumont, M. Gulea
Tetrahedron Letters, 2008, 49, 4329-4332.
Thiazolinium and imidazolium chiral ionic liquids derived from natural amino acid
derivatives
D Bregeon, J. Levillain, F. Guillen, J.-C. Plaquevent, A.-C. Gaumont
Amino Acids, 2008, 35, 175-184 (DOI 10.1007/s00726-007-057-3).
(N,N) versus (N,S) chelation of palladium in asymmetric allylic substitution using
bis(thiazoline) ligands; a theoretical and experimental study
A. Betz, L. Yu, M. Reiher, A.-C. Gaumont, P.-A. Jaffrès, M. Gulea
Journal of Organometallic Chemistry, 2008, 693, 2499-2508.
Diastereoselective Michael additions to alpha,beta-unsaturated alpha sulfinyl phosphonates in the thiolane series
P. Lyzwa, A. Jankowiak, M. Mikolajczyk, P. Kielbasinski, A. Betz, P.-A. Jaffrès, A.-C. Gaumont, M. Gulea
Tetrahedron Letters, 2007, 48, 351-355.
Palladium-Catalyzed C-P Coupling Reactions between Vinyl Triflates and Phosphine – Boranes:
Efficient Access to Vinylphosphine-Boranes
D. Julienne, J.-F. Lohier, O. Delacroix, A.-C. Gaumont
Journal of Organic Chemistry, 2007, 72, 2247-2250.
Enantiomerically Pure Thiazolinium and Imidazolium Low Melting Point Salts Prepared from
alpha-Aminoacids
D. Brégeon, J. Levillain, F. Guillen, J.-C. Plaquevent, A.-C. Gaumont
ACS Book Series, 2007, 19, 246-258.
Development of new SILP catalysts using chitosan as support
J Baudoux, K Perrigaud, P.-J. Madec, A.-C. Gaumont, I. Dez
Green Chemistry, 2007, 9, 1346-1351.
INTERNATIONALES
Dr K. Glinel, Univ de Louvain la Neuve, Belgique
Pr R. Stephenson, Université de Norwich (UEA)
Pr C. Richards, Université de Norwich (UEA)
Pr Herbert Mayr, LMU-Munich
Pr Ryan Gilmour, Université de Munster
G. Evano, CPCO, Université Libre de Bruxelles
Thomas Bürgi (Genève, Suisse)
Graham J. Bodwell (Terre-Neuve, Canada)
NATIONALES
Dr M. Taillefer, ENSC Montpellier
Dr J.C. Plaquevent, Univ de Toulouse
Dr C. Lepetit, LCC, Université de Toulouse
Dr M. Mauduit, Univ de Rennes
Dr J. Maddaluno, COBRA, Université de Rouen
Dr A. Harisson-Marchand, COBRA, Université de Rouen
Pr H. Oulyadi, COBRA, Université de Rouen
Dr J. -F. Brière, COBRA, Université de Rouen
Dr V. Levacher, COBRA, Université de Rouen
Dr I. Chataignier, Université de Rouen
Dr. K. Katyeyeva, PBS, Université de Rouen
Dr J. Koselka, Université Paris V
Pr H. Gerard, LCT, Paris VI
Dr E. Leroy, Université de Nantes-St Nazaire
Pr A. Tatibouët, ICOA (Orléans)
Dr V. Dalla, IRCOM, Université du Havre
Dr S. Thibaudeau, IC2MP, Université de Poitiers
Dr C. Caplat, BOREA, Cherbourg en Cotentin
Dr H. Gueuné, CORRODYS, Cherbourg en Cotentin
LOCALES
Dr. I. Dez (LCMT)